Phase II Started for a Study Towards the Prevention of Cerebral Palsy
A research group led by Haruo Shintaku, a specially appointed professor of the Graduate School of Medicine, Osaka City University, overseeing a course endowed with disability and regenerative medicine, has safely completed phase I of an autologous umbilical cord blood treatment for hypoxic Ischemic Encephalopathy (HIE). Phase II※1 has started on November 12, 2020.
※1 Clinical research based on the Act on the Safety of Regenerative Medicine.?
Background?
Severe asphyxia is a condition in which breathing and pulse are weakened when a baby is born. Perinatal hypoxic ischemic encephalopathy, which is the main cause, is a brain disorder that happens when there is a blockage of blood flow to the brain at birth, causing brain nerve cells to fall into hypoxia and hypoglycemia. It occurs at a rate of 1 to 6 out of every 1000 live births. Even with hypothermia therapy, which is considered to be an effective treatment for babies, half of them develop serious secondary complications, with the muscle rigidity and difficulty in exercising typical of cerebral palsy being a main one.?
Research Summary?
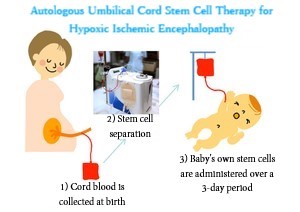
The 6 cases required for the Phase I study to verify safety have been confirmed, making it the first clinical research to confirm safety of the treatment. Next, to confirm the feasibility and effects of treatment, Phase II of clinical trials has started in November. Phase II requires 15 cases, which is more than double that of Phase I. It has started in 9 facilities※2, with plans to increase the number to 18.
※2 Iwate Medical University, Osaka City General Hospital of Osaka City University, Kurashiki Central Hospital, Tokyo Metropolitan Children's Medical Center, Dokkyo Medical University, Nagoya University, Nihon University, Yodogawa Christian Hospital.

Haruo Shintaku
Comment from the researcher?
In the phase II of this study, we have asked StemCell Institute Inc., a private cord blood bank, to aid facilities that do not have an umbilical cord blood stem cell separator, by receiving shipments of cord blood and preparing them for use in autologous umbilical cord stem cell therapy. If this is successful, more hospitals will be able to carry out this treatment.
?
Reference
◆ StemCell Institute website https://www.stemcell.co.jp/
◆ Research results so far
? Started clinical research on cord blood stem cell therapy for newborns with hypoxic encephalopathy (published on May 12, 2015)
? The first case in Japan of autologous cord blood stem cell therapy for neonatal hypoxic-ischemic encephalopathy: A boy was released in good health (posted May 29, 2015)
? AMED 2014 start issues and results report
? Autologous cord blood stem cell therapy for neonatal hypoxic-ischemic encephalopathy-Phase I study completed, towards phase II (Posted on February 23, 2018)
Funding
Financial support received as the research and development project "Research on autologous cord blood stem cell therapy for hypoxic-ischemic encephalopathy" in AMED's " Research Project for Practical Applications of Regenerative Medicine" (implementation period: 2018 to 2020).

